Team:Alberta-North-RBI E/genetics
From 2012e.igem.org
(→Genetics) |
|||
| Line 1,071: | Line 1,071: | ||
</tr> | </tr> | ||
</table> | </table> | ||
| + | </center> | ||
</center> | </center> | ||
</html> | </html> | ||
Revision as of 03:11, 23 October 2012
Genetics
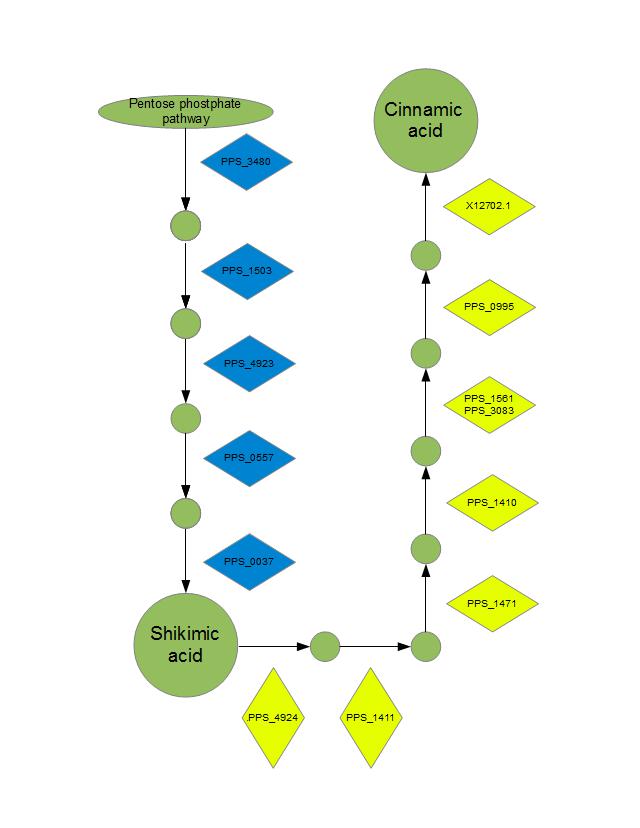
We plan to use a single metabolic pathway for the conversion of glucose to aromatic chemicals. We are currently engineering a Pseudomonas putida strain containing a “switch” system enabling the manufacture of either shikimic acid (SA), cinnamic acid (CA) as a major product. Future embodiments of this strain will contain additional directing mechanisms whereby a multitude of derivative chemicals can be favored.

Our metabolic engineering strategy is based on the invention of Dr. John Frost (US Patent No. 5168056, 6613552). The enzymatic activities leading to the production of SA will be enhanced by the addition of extra-chromosomal copies of endogenous genes modified such that expression is constitutive . Where necessary, these genes will be further modified through site-directed mutagenesis to abolish feedback regulation. To enhance the yield of our desired products, two additional categories of changes will be made to our host strain: (1) Enzymatic activities which direct metabolites away from the desired pathway will be abolished by removing the underlying endogenous genes and (2) transporter proteins responsible for re-uptake of SA or CA from the medium will be removed in the same manner.
Enzymatic activities which lead from SA to phenylalanine (Phe) will be abolished by removing the underlying endogenous genes. These endogenous genes, in addition to a codon-optimized Rhodosporidium toruloides gene encoding for a phenylalanine ammonia-lysase (PAL) enzyme, will be re-introduced at an extra-chromosomal locus as modified genes containing inducible promoter/repressor “switches” allowing us to direct metabolism toward CA at will. The “switch” mechanism will require “cis” DNA elements immediately upstream of each enzyme’s coding sequence (CDS). In addition, each regulatory system (ie. induction, repression) will require a constitutively-regulated “trans” element encoded at a different locus which produces the protein component.
Our genetic switch will be designed to tightly regulated gene expression. This will be accomplished by using both an inducer module and a repressor module so that strong expression of the regulated gene is only possible when a dual signal is given. One signal will cause an “inducer protein” to bind a cis regulatory element upstream of the promoter, where it can recruit cell machinery to initiate transcription. This signal alone, however, would be insufficient to allow expression. A repressor cis element located between the inducer cis element and the promoter is normally bound by a “repressor protein” which prevents movement of the transcription machinery down the gene. A second signal will cause the “repressor protein” to dissociate from it's cis element, thus clearing the transcription machinery's path and allowing expression. The exact details of this regulatory system are still under investigation – the design of this system may become a focus of next year's Alberta North iGEM team.
|
Part name |
Gene/Locus |
Source |
Note |
|
Constitutive promoter |
n/a |
Synthetic |
General part |
|
Inducible promoter (cis) |
n/a |
Synthetic |
General part |
|
Inducible promoter (trans) |
n/a |
Synthetic |
General part |
|
Inducible repressor (cis) |
n/a |
Synthetic |
General part |
|
Inducible repressor (trans) |
n/a |
Synthetic |
General part |
|
Ribosome binding site |
n/a |
Synthetic |
General part |
|
Transcription terminator |
n/a |
Synthetic |
General part |
|
Transketolase |
PPS_3480 |
P. putida |
Constitutively upregulated |
|
DAHP synthase |
PPS_1503 |
P. putida |
Constitutively upregulated, Thr326Pro
= feedback insensitive |
|
DHQ synthase |
PPS_4923 |
P. putida |
Constitutively upregulated |
|
DHQ dehydratase |
PPS_0557 |
P. putida |
Constitutively upregulated |
|
Shikimate dehydrogenase |
PPS_0037 |
P. putida |
Constitutively upregulated |
|
Shikimate kinase |
PPS_4924 |
P. putida |
Removed and reintroduced as inducible |
|
3-phosphoshikimate 1-carboxyvinyltransferase |
PPS_1411 |
P. putida |
Removed and reintroduced as inducible |
|
Chorismate synthase |
PPS_1471 |
P. putida |
Removed and reintroduced as inducible |
|
Chorismatemutase |
PPS_1410 |
P. putida |
Removed and reintroduced as inducible |
|
Aromatic amino acid aminotransferase |
PPS_1561 |
P. putida |
Removed and reintroduced as inducible |
|
Aromatic amino acid aminotransferase |
PPS_3083 |
P. putida |
Removed and reintroduced as inducible |
|
Histidinol-phosphate aminotransferase |
PPS_0995 |
P. putida |
Removed and reintroduced as inducible |
|
Phenylalanine ammonia-lysase |
|
R. toruloides |
Introduced as inducible |
|
Shikimate kinase |
PPS_4924 |
P. putida |
Removed and reintroduced as inducible |
|
3-phosphoshikimate 1-carboxyvinyltransferase |
PPS_1411 |
P. putida |
Removed and reintroduced as inducible |
|
Chorismate synthase |
PPS_1471 |
P. putida |
Removed and reintroduced as inducible |
|
Chorismatemutase |
PPS_1410 |
P. putida |
Removed and reintroduced as inducible |
|
Aromatic amino acid aminotransferase |
PPS_1561 |
P. putida |
Removed and reintroduced as inducible |
|
Aromatic amino acid aminotransferase |
PPS_3083 |
P. putida |
Removed and reintroduced as inducible |
|
Histidinol-phosphate aminotransferase |
PPS_0995 |
P. putida |
Removed and reintroduced as inducible |
|
Pyrroloquinolinequinone |
PPS_3064 |
P. putida |
Removed |
|
Anthranilate synthase component I |
PPS_0413 |
P. putida |
Removed |
|
Anthranilate synthase component II |
PPS_0416 |
P. putida |
Removed |
|
Para-aminobenzoate synthase subunit I |
PPS_1911 |
P. putida |
Removed |
|
Shikimate transporter |
shiA |
P. putida |
Removed |
|
Cinnamic acid transporter |
|
|
Removed |
 "
"