Team:Alberta-North-RBI E/projectsolution
From 2012e.igem.org
| Line 10: | Line 10: | ||
=='''OUR SOLUTION'''== | =='''OUR SOLUTION'''== | ||
| + | |||
| + | As technologies continue to improve, there has been increasing interest in exploring the use of biomass as a renewable resource. Biomass is typically a by-product of an industrial process and considered waste. In particular, the paper sludge produced by paper recycling plants is presently disposed of via landfill or burnt. This is a significant source of potentially exploitable cellulose that can not only be used to produce high-value chemicals but will also have a positive impact on waste management. Additionally, paper sludge is an attractive feedstock compared to other lignocellulosic biomass because is processed prior to its utilization and requires no pre-treatment as a result. | ||
| + | |||
| + | |||
| + | Many studies have concentrated on the conversion of the cellulose in the paper waste to different types of value-added chemicals. Primarily, it has been considered for conversion to ethanol (Yamshita et al. 2006; Vamvuka et al. 2009; Kang et al. 2010, 2011) and lactic acid (Marques et al. 2008; Mukhopadhyay 2009). Our business plan focuses on the production of aromatics from this cellulose. Aromatics represent a potentially lucrative chemical endpoint, having high price per unit mass and a sustainable market in both the pharmaceuticals and cosmetics industries. | ||
| + | |||
| + | |||
| + | Our company’s proposed process has two parts: in the first, cellulose from waste sludge from recycling plants is converted into glucose; in the second, glucose from the first part is used as a feedstock for the production of aromatic chemicals. In the glucose-aromatic conversion, we plan to use only a single metabolic pathway with “on and off switches” at each “step”. This gives us the freedom to produce any intermediary compounds in addition to the natural end product. The current proposed pathway will allow us to produce either shikimate or cinnamic acid derivatives using the method described above. Possible switch activators include temperature pH, or the addition of an inhibiting chemical such as phosphate. | ||
| + | |||
| + | |||
| + | Our solution encompasses two sections: | ||
| + | |||
| + | |||
| + | Process Design | ||
| + | |||
| + | |||
| + | Genetics | ||
=='''Genetics'''== | =='''Genetics'''== | ||
Revision as of 01:47, 11 October 2012

OUR SOLUTION
As technologies continue to improve, there has been increasing interest in exploring the use of biomass as a renewable resource. Biomass is typically a by-product of an industrial process and considered waste. In particular, the paper sludge produced by paper recycling plants is presently disposed of via landfill or burnt. This is a significant source of potentially exploitable cellulose that can not only be used to produce high-value chemicals but will also have a positive impact on waste management. Additionally, paper sludge is an attractive feedstock compared to other lignocellulosic biomass because is processed prior to its utilization and requires no pre-treatment as a result.
Many studies have concentrated on the conversion of the cellulose in the paper waste to different types of value-added chemicals. Primarily, it has been considered for conversion to ethanol (Yamshita et al. 2006; Vamvuka et al. 2009; Kang et al. 2010, 2011) and lactic acid (Marques et al. 2008; Mukhopadhyay 2009). Our business plan focuses on the production of aromatics from this cellulose. Aromatics represent a potentially lucrative chemical endpoint, having high price per unit mass and a sustainable market in both the pharmaceuticals and cosmetics industries.
Our company’s proposed process has two parts: in the first, cellulose from waste sludge from recycling plants is converted into glucose; in the second, glucose from the first part is used as a feedstock for the production of aromatic chemicals. In the glucose-aromatic conversion, we plan to use only a single metabolic pathway with “on and off switches” at each “step”. This gives us the freedom to produce any intermediary compounds in addition to the natural end product. The current proposed pathway will allow us to produce either shikimate or cinnamic acid derivatives using the method described above. Possible switch activators include temperature pH, or the addition of an inhibiting chemical such as phosphate.
Our solution encompasses two sections:
Process Design
Genetics
Genetics
Our Microscopic Friend
We intend to engineer a Pseudomonas putida strain containing a “switch” system enabling the manufacture of either shikimic acid (SA) or cinnamic acid (CA) as a major product. Future embodiments of this strain will contain additional directing mechanisms whereby a multitude of derivative chemicals can be favored.
The enzymatic activities leading to the production of SA will be enhanced by the addition of extra-chromosomal copies of endogenous genes modified such that expression is constitutive. Where necessary, these genes will be further modified through site-directed mutagenesis to abolish feedback regulation. Optimization of metabolism to SA is based on the invention(s) described in US patents 5168056 and 6613552.
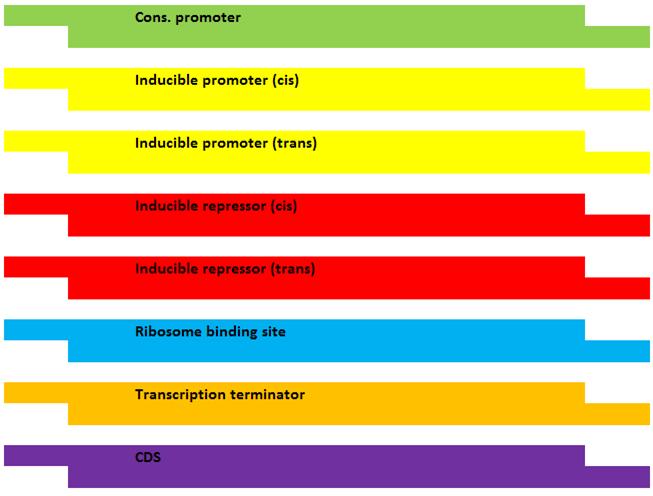
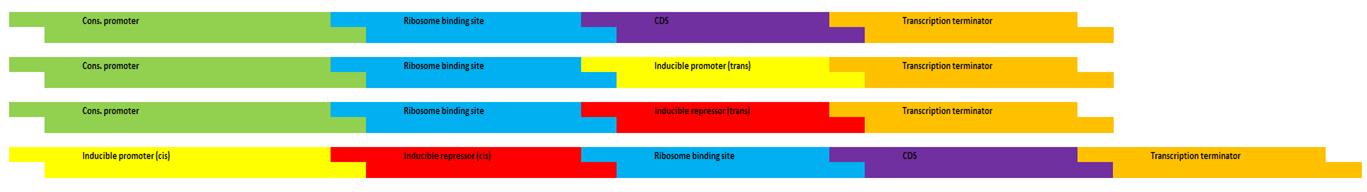
Enzymatic activities which lead from SA to phenylalanine (Phe) will be abolished by removing the underlying endogenous genes. These endogenous genes, in addition to a codon-optimized Rhodosporidium toruloides gene encoding for a phenylalanine ammonia-lysase (PAL) enzyme, will be re-introduced at an extra-chromosomal locus as modified genes containing inducible promoter/repressor “switches” allowing us to direct metabolism toward CA at will. The “switch” mechanism will require “cis” DNA elements immediately upstream of each enzyme’s coding sequence (CDS). In addition, each regulatory system (ie. induction, repression) will require a constitutively-regulated “trans” element encoded at a different locus which produces the protein component.
To enhance the yield of our desired products, two additional categories of changes will be made to our host strain. Enzymatic activities which direct metabolites away from the desired pathway will be abolished by removing the underlying endogenous genes and transporter proteins responsible for re-uptake of SA or CA from the medium will be removed in the same manner.
 "
"