Team:Amplino/Solution/Competition
From 2012e.igem.org
(→Competition) |
(→Competition) |
||
| (3 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
==Competition== | ==Competition== | ||
| - | + | Organizations such as UNITAID or the World Health Organisation publish yearly documents on the competitive landscape of malaria diagnostics. The UNITAID report can be found [http://www.unitaid.eu/images/marketdynamics/publications/UNITAID%20Malaria%20Diagnostics%20Market%20Landscape_2012.pdf here]. | |
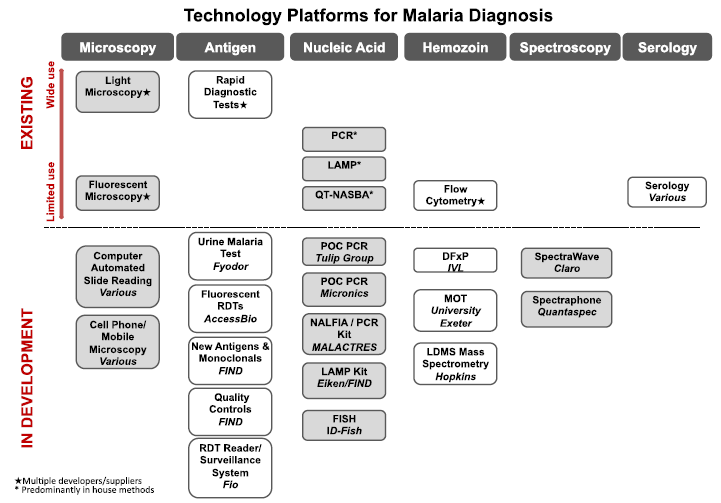
| - | + | A great number of malaria diagnostics methods already exists, but most of them are still constrained inside laboratories, as shown in the image below (image courtesy of UNITAID) | |
| - | + | [[File:UNITAID_Malaria_TechnologyLandscape.png]] | |
| - | + | ||
| - | + | Amplino is about using PCR technologies outside the lab (known as point-of-care). The higher sensitivity will be beneficial especially for pregnant woman. Additionally, PCR allows to discover the kind of malaria species that is involved, which partly determines how lethal the disease is. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Latest revision as of 15:27, 21 October 2012
Competition
Organizations such as UNITAID or the World Health Organisation publish yearly documents on the competitive landscape of malaria diagnostics. The UNITAID report can be found [http://www.unitaid.eu/images/marketdynamics/publications/UNITAID%20Malaria%20Diagnostics%20Market%20Landscape_2012.pdf here].
A great number of malaria diagnostics methods already exists, but most of them are still constrained inside laboratories, as shown in the image below (image courtesy of UNITAID)
Amplino is about using PCR technologies outside the lab (known as point-of-care). The higher sensitivity will be beneficial especially for pregnant woman. Additionally, PCR allows to discover the kind of malaria species that is involved, which partly determines how lethal the disease is.
 "
"