From 2012e.igem.org
(Difference between revisions)
|
|
| Line 4: |
Line 4: |
| | A great number of malaria diagnostics methods already exists, but most of them are still constrained inside laboratories, as shown in the image below (image courtesy of UNITAID) | | A great number of malaria diagnostics methods already exists, but most of them are still constrained inside laboratories, as shown in the image below (image courtesy of UNITAID) |
| | [[File:UNITAID_Malaria_TechnologyLandscape.png]] | | [[File:UNITAID_Malaria_TechnologyLandscape.png]] |
| - |
| |
| - | In the table below we compare a great number of competitors in the field of medical diagnostics. Not all of them necessarily offer products for Malaria, but we feel the technology they posses might be applicable.
| |
| - |
| |
| - | When judging their potential threat, we used a scoring system based on our business and design requirements list.
| |
| - |
| |
| - |
| |
| - |
| |
| - | <html>
| |
| - | <table>
| |
| - | <tr>
| |
| - | <td>Name</td>
| |
| - | <td>Company</td>
| |
| - | <td>Type</td>
| |
| - | <td>Speed</td>
| |
| - | <td>Sample</td>
| |
| - | <td>Cost</td>
| |
| - | <td>Requirements</td>
| |
| - | <td>Application</td>
| |
| - | <td>Picture</td>
| |
| - | </tr>
| |
| - | <tr>
| |
| - | <td><a href="http://www.axelabiosensors.com/real-time-immunoassays/dotlab-system.php">dotLab</a></td>
| |
| - | <td>Alexa</td>
| |
| - | <td>real time Immuno Assay</td>
| |
| - | <td>15 - 90 min</td>
| |
| - | <td>Blood</td>
| |
| - | <td>tbd</td>
| |
| - | <td>100-240V, 250W</td>
| |
| - | <td></td>
| |
| - | <td><img src="https://static.igem.org/mediawiki/2012e/a/a5/AMPLINO-dotLab.jpg" width=250 /></td>
| |
| - | </tr>
| |
| - | <tr>
| |
| - | <td><a href="http://dfa.org/">Patterned Paper</a></td>
| |
| - | <td>Diagnostics for All</td>
| |
| - | <td>Immuno Assay</td>
| |
| - | <td>10 min</td>
| |
| - | <td>Blood</td>
| |
| - | <td>tbd</td>
| |
| - | <td>none</td>
| |
| - | <td>Liver test in development</td>
| |
| - | <td><img src="https://static.igem.org/mediawiki/2012e/d/d2/AMPLINO-dfa.png" width=250 /></td>
| |
| - | </tr>
| |
| - | <tr>
| |
| - | <td><a href="http://www.inbios.com/rapid-tests/malaria-detect-pfpv">Malaria Pf/Pv Detect</a></td>
| |
| - | <td>InBios</td>
| |
| - | <td>Immuno Assay</td>
| |
| - | <td>10 min</td>
| |
| - | <td>Serum</td>
| |
| - | <td>tbd</td>
| |
| - | <td>Centrifuge</td>
| |
| - | <td>Malaria</td>
| |
| - | <td><img src="https://static.igem.org/mediawiki/2012e/1/11/AMPLINO-InBios.jpg" width=250 /></td>
| |
| - | </tr>
| |
| - | <tr>
| |
| - | <td><a href="http://www.orasure.com/products-infectious/products-infectious-oraquick.asp">OraQuick</a></td>
| |
| - | <td>OraSure</td>
| |
| - | <td>Immuno Assay</td>
| |
| - | <td>40 min</td>
| |
| - | <td>Salvia</td>
| |
| - | <td>tbd</td>
| |
| - | <td>none</td>
| |
| - | <td>HIV</td>
| |
| - | <td><img src="https://static.igem.org/mediawiki/2012e/7/72/AMPLINO-OraQuick.jpg" height=170 /></td>
| |
| - | </tr>
| |
| - | <tr>
| |
| - | <td><a href="http://www.clarosdx.com/technology.php">Handheld Analyzer</a></td>
| |
| - | <td>Claros</td>
| |
| - | <td>Immuno Assay</td>
| |
| - | <td>15 min</td>
| |
| - | <td>Blood</td>
| |
| - | <td>$100 per device</td>
| |
| - | <td>none</td>
| |
| - | <td>HIV, syphilis and anemia in development</td>
| |
| - | <td><img src="https://static.igem.org/mediawiki/2012e/a/aa/Amplino-Claros.jpg" height=170 /></td>
| |
| - | </table>
| |
| - | </html>
| |
Revision as of 15:56, 20 October 2012
Competition
Organizations such as Unitaid or the World Health Organisation publish yearly documents on the competitive landscape of malaria diagnostics. The UNITAID report can be found [http://www.unitaid.eu/images/marketdynamics/publications/UNITAID%20Malaria%20Diagnostics%20Market%20Landscape_2012.pdf here].
A great number of malaria diagnostics methods already exists, but most of them are still constrained inside laboratories, as shown in the image below (image courtesy of UNITAID)
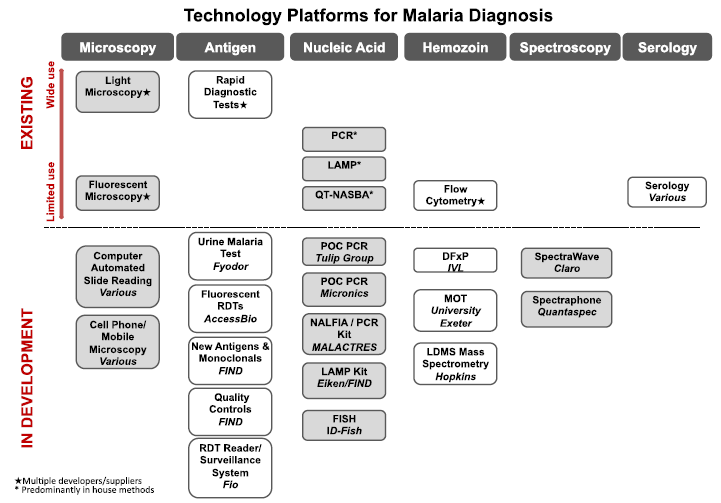

 "
"